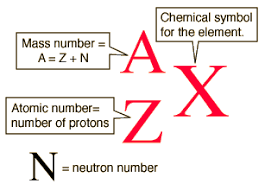Atomic Number and Mass Number

ATOMIC NUMBER: Protons are present in the nucleus of an atom. It is the number of protons of an atom. which determines its atomic number. It is denoted by ‘Z’. All atoms of an element have the same atomic number, Z. In fact, elements are defined by the number of protons they posses. For hydrogen, Z=1, because in hydrogen atom, only one proton is present in the nucleus. Similarly, for carbon, Z=6. Therefore, the atomic number is defined as the total number of protons present in the nucleus of an atom.
MASS NUMBER: After studying the properties of the sub- atomic particles of an atom, we can conclude that mass of an atom is practically dur to protons and neutrons alone. These are present in the nucleus of an atom. Hence protons and neutrons are also called nucleus. Therefore, the mass of an atom resides in its nucleus. For ex – Mass of carbon 12u because it has 6 protons and 6 neutrons, 6u + 6+ = 12u. Similarly , the mass of aluminium is 27u ( 13 protons + 14 neutrons ). The mass number is defined as the sum of the total number of protons and neutrons present in the nucleus of an atom. In the notation fort an atom, the atomic number, mass number and symbol of the element are to be written as