Dalton’s Atomic theory

According to Dalton’s Atomic theory, all matter, whether an element, a compound or a mixture is composed of small particles called atoms.
Six postulates of Dalton’s Atomic theory:
- All matter is made of very tiny particles called atoms.
- Atoms are indivisible particles, which cannot be created or destroyed in a chemical reaction.
- Atoms of a given element are identical in mass and chemical properties
- Atoms of different elements have different masses and chemical properties.
- Atoms combine in the ratio of small whole numbers to form compounds.
- The relative number and kinds of atoms are constant in a given compound.
Atoms: Atoms are building blocks of all matter.
- According to modern Atomic theory, an element is the smallest particle of an element which takes part in chemical reaction.
- Atoms are very small and which can’t be seen even through very powerful microscope.
- Atomic radius is measured in nanometres.
- 1nm=10-9m
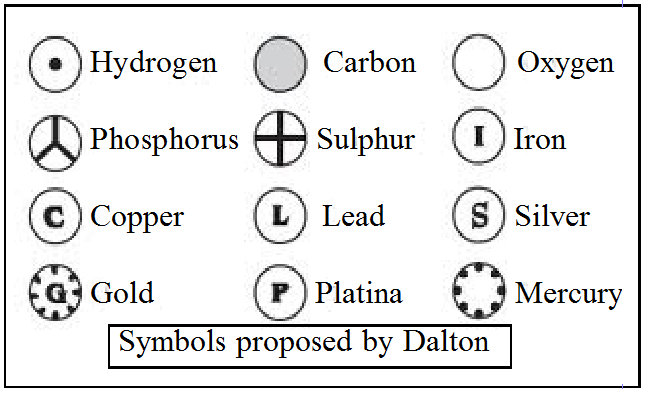
Modern day symbols of Atoms of different elements.
- Dalton was the first scientist to use the symbols for elements in a very specific sense.
- Berzilius suggested that the symbols of elements be made from one or two letters of the name of the element.