Neutrons

In 1932, J. Chadwick discovered another sub-atomic particle which had no charge and a mass nearly equal to that of a proton. It was eventually named as neutron. Neutrons are present in the nucleus of all atoms, except hydrogen. In general, a neutron is represented as ‘n’. The mass of an atom is therefore given by the sum of the masses of protons and neutrons present in the nucleus.
| particles | Explorer | mass | charge |
| Electron | J.J Thomson(1897) | 9.1*10-31kg | -Ve |
| Proton | Rutherford(1917) | 1.6725*10-27kg | +Ve |
| Neutron | Chadwick(1932) | 1.6748*10-27kg | No charge |
How are Electrons Distributed in Different Orbits (Shells)?
The distribution of electrons into different orbits of an atom was suggested by Bohr and Bury. The following rules are followed for writing the number of electrons in different energy levels or shells:
- The maximum number of electrons present in a shell is given by the formula 2n2, where ‘n’ is the orbit number or energy level index, 1,2,3,…. Hence the maximum number of electrons in different shells are as follows: – First orbit or K-shell will be = 2×12= 2, Second orbit or L-shell will be = 2×22=8 Third shell or M-shell will be = 2×32 = 18, Fourth orbit or N-shell will be = 2×42 = 32, and so on.
- The maximum number of electrons that can be accommodated in a given shell, unless the inner shells are filled. That is, the shells are filed in a step-wise manner. Atomic structure of the first eighteen elements is shown in image-
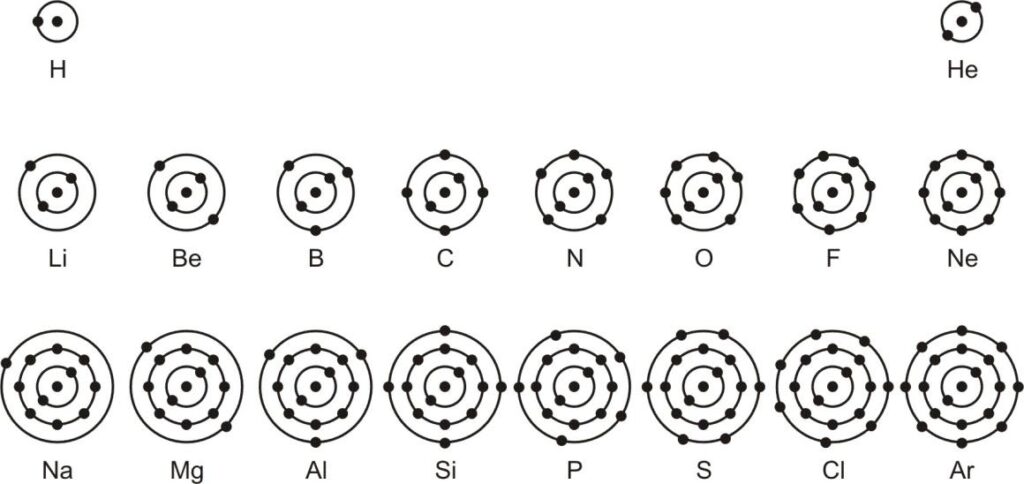
fig: Schematic atomic structure of the first eighteenth elements


