Molecular Mass

The molecular mass of a substance is the sum of the atomic masses of all the atoms in a molecule of the substance.
Ex- Atomic mass of H2O
= 2*Atomic mass of Hydrogen + 1*Atomic mass of Oxygen
= 2*1 + 1*16
= 2 + 16
= 18 u
Formula Unit Mass
- The formula unit mass of a substance is a sum of the atomic masses of all atoms in a formula unit of a compound.
- It is calculated in the same manner of molecules mass.
Ex- Calculate the formula unit mass of CaCl2.
= Atomic mass of Ca + 2*Atomic mass of Chlorine
= 40 + 2*35.5
= 40 + 71
= 111u
Ions: An ion may be defined as an atom or group of atoms having positive or negative charge.
- Some positively charged ions : Na+, K+, Ca2+, Al3+
- Some negatively charged ions.
Cl– ( Chlorine ion), S2-( Sulphide ion)
We can classify ions in two types:
- Simple ions
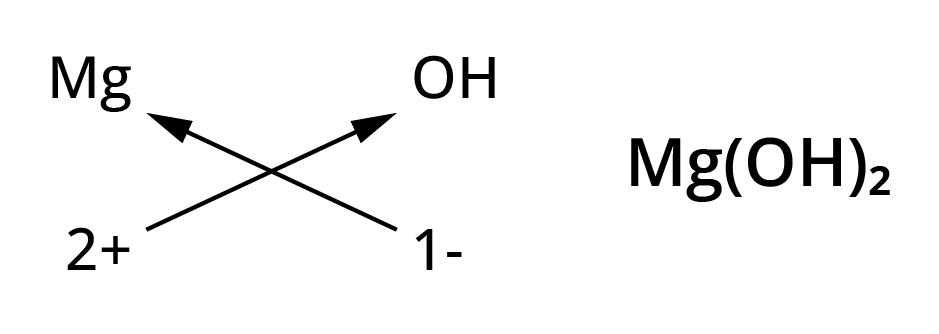
- Mg2+( Magnesium ion)
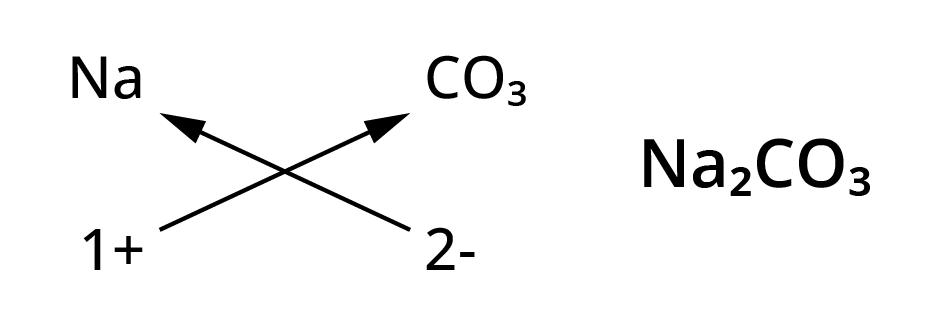
- Na+ ( Sodium ion)
- Cl– ( Chloride ion)
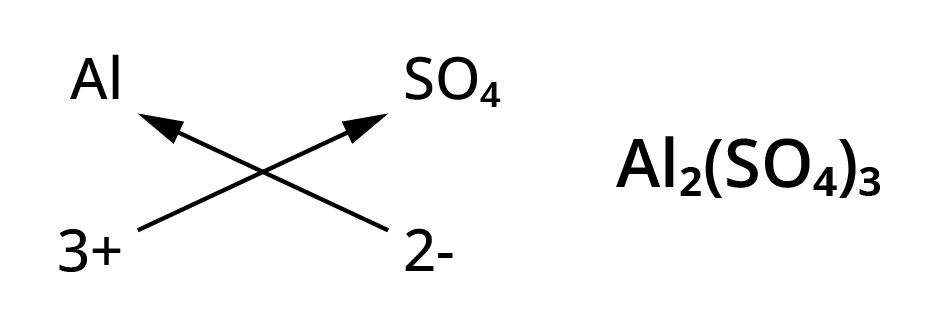
- Al3+ ( Aluminium ion)
- Compound ions
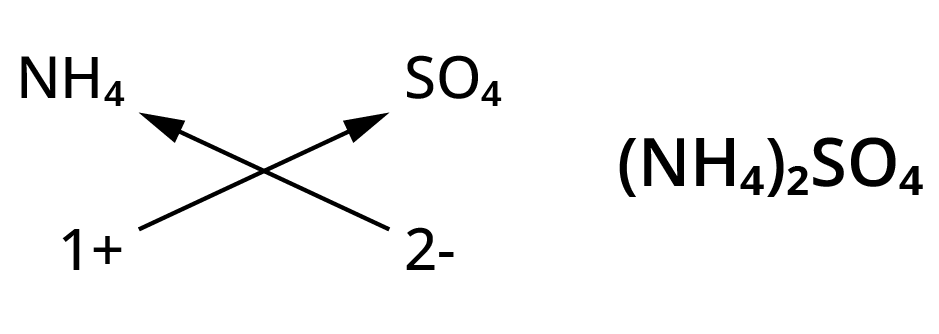
- NH4- (Ammonium ion)
- CO32-(Carbonate ion)
- SO42-(Sulphate ion)
- OH– ( Hydroxide ion)
Chemical formulae of ionic compounds(polyatomic)