Ions

- Compounds composed of metals and non-metals contain charged species. The charged species are known as ions.
- A negatively charged ion is called an “anion” and a positively charged ion is called a ” cation”. Ex-NaCl where Na+ and Cl–
- Ions may consist of a single charged atom or a group of atoms that have a net charge on them.
Polyatomic ion: A group of atoms carrying a charge is known as a polyatomic ion.
Chemical Formula: The chemical formula of a compound is a symbolic representation of its composition.
Characteristics of chemical formulae:
- The valencies or charges on the ion must balance.
- When a compound is formed of metal and non-metal, symbol of metal comes first. Ex- CaO, NaCl, CuO.
- When polyatomic ions are used, the ions are enclosed in brackets before writing the number to show the ratio. Ex- Ca(OH)2, (NH4)2SO4
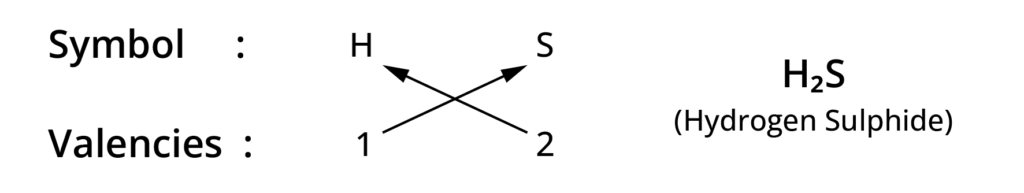
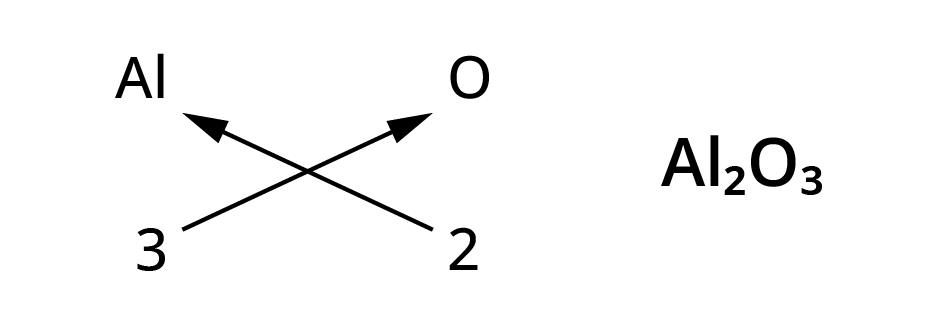
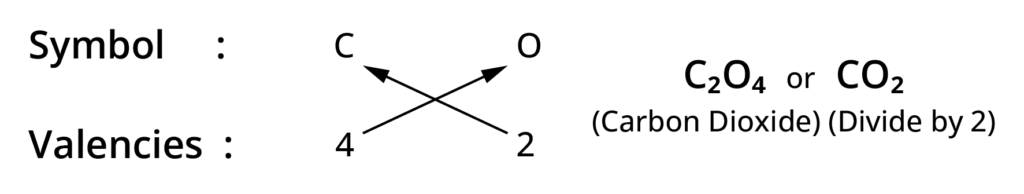
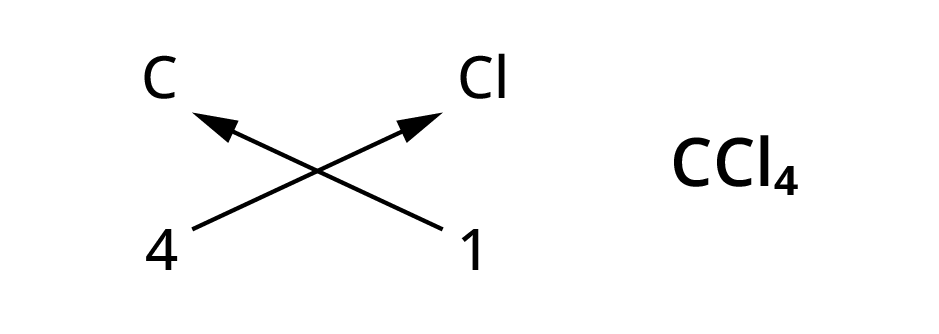
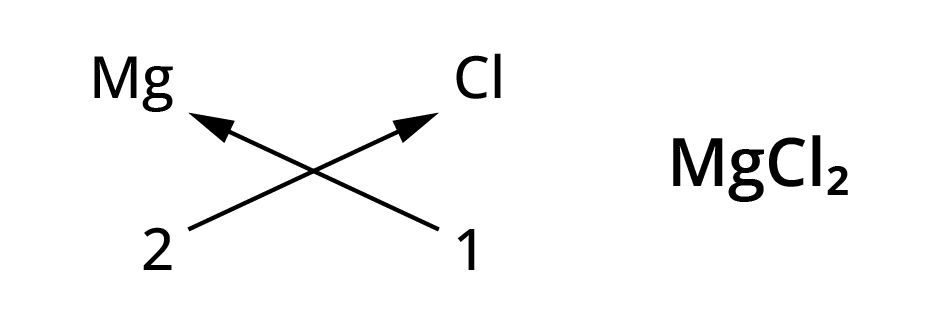
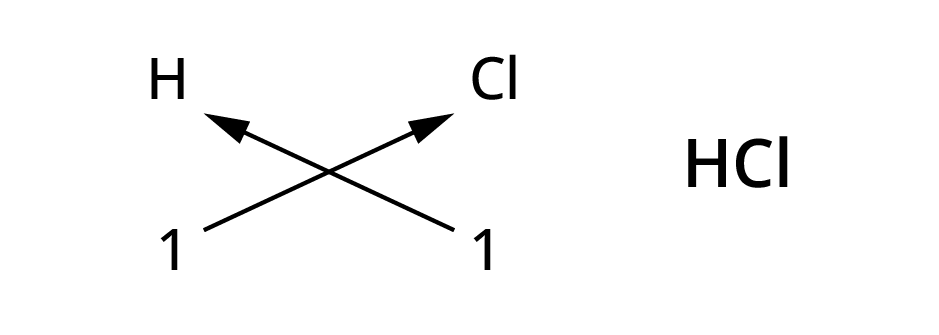
Rules for writing chemical formulae
- We first write symbol of element which form compound.
- Below the symbol of each element, we should write their valency.
- Now cross over the valencies of combining atoms.
- With first atom, we write the valency of second atom ( as a subscript)
- With second atom, we write the valency of first atom( Subscript).